What are carbanions? How are these generated? Discuss the relative stabilities of primary, secondary and tertiary carbanions.
Carbanions: Carbanions may be defined as negatively charged ions, in which carbon is having a negative charge and it has eight electrons in the valence shell. For example,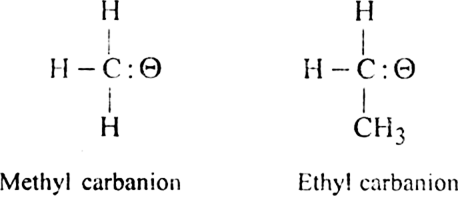
Carbanions are very short-lived and highly reactive species.
Generation of carbanion: These are mostly generated in the presence of a base by heterolytic cleavage. 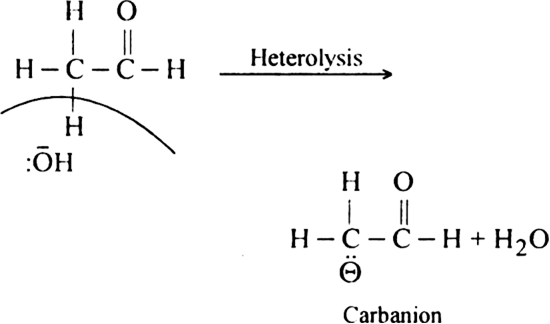
Stability of carbanions. Amongst primary (1°) secondary (2°) and tertiary (3°) carbanions, 1° is the most stable.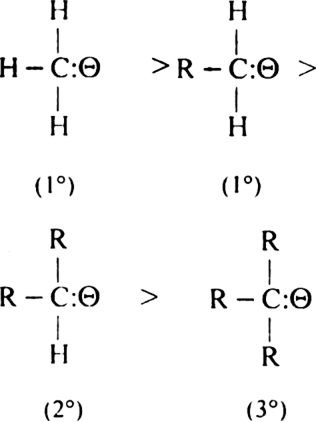
The above stability order can be explained by inductive effect. Alkyl group has +I effect. Thus electron releasing group intensifies the negative charge on the carbon atom and destabilises the carbanion. In 3° carbanion due to the presence of three alkyl groups with +I effect, a negative charge is intensified on the carbon atoms and the carbanion gets destabilised. So this is the least stable carbanion. Hence primary carbanion with one alkyl group is, therefore, more stable than secondary (with two alkyl groups) which in turn is more stable than tertiary (with three alkyl groups). In methyl carbanion, H has not any appreciable inductive effect, so it is most stable.
