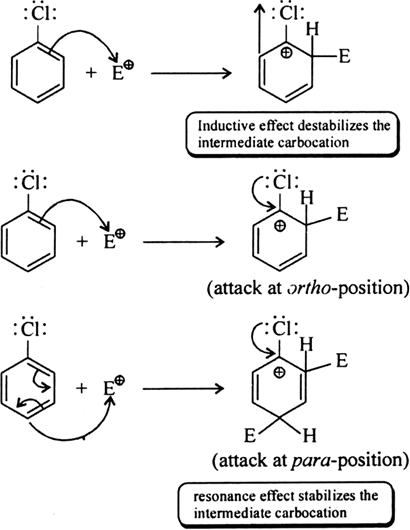
Through resonance, halogen tends to stabilise the carbocation and the effect is more pronounced at ortho- and para- positions. The
inductive effect is stronger than resonance and causes net electron withdrawal and thus causes net deactivation. The resonance effect tends to oppose the inductive effect for the attack at ortho- and parapositions and hence makes the deactivation less for ortho- and paraattack.
Reactivity is thus controlled by the stronger inductive effect and orientation is controlled by resonance effect.
