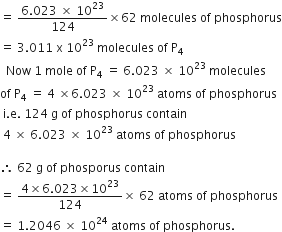
How many atoms and molecules of phosphorus are present in 62 g of phosphorus (P4)?
Molecular formula of phosphorus = P4
Gram molecular mass of phosphorus (P4)
= 4 x 31 = 124 g
124 g of phosphorus contains 6.023 x 1023 molecules of phosphorus
![]() 62 g of phosphorus would contain
62 g of phosphorus would contain