(a) Write the hybridization and shape of the following complexes:
(i) [CoF6]3–
(ii) [Ni (CN)4]2–
(Atomic number: Co = 27, Ni = 28)
(b) Out of NH3 and CO, which ligand forms a more stable complex with a transition metal and why?
(a)
(i) The atomic number of Co is 27 and its valence shell electronic configuration is 3d74s2.
Co is in +3 oxidation state in the complex [CoF6]3-.

Hence, [CoF6]3- is sp3d2 hybridized and it is octahedral in shape.
(ii) The atomic number of Ni is 28 and its valence shell electronic configuration is 3d84s2.
Ni is in +2 oxidation state in the complex [Ni(CN)4]2–.
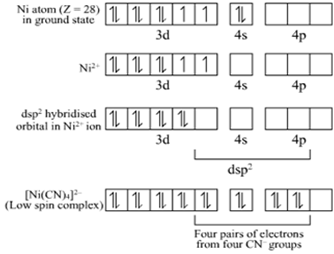
Hence, [Ni(CN)4]2– is dsp2 hybridized and it is square planar in shape.
(b) Out of NH3 and CO, CO ligand forms a more stable complex with transition metal because the metal- carbon bonds in metal carbonyls have both and characters .A bond is formed when carbonyl carbon donates a lone pair of electrons to the vacant orbital of the metal. A bond formed by the donation pair of electrons from filled metal d orbital into the vacant anti- bonding orbital ( also known as back bonding of the carbonyl group). And sigma bond strengthen the pi bond vice-versa. Thus, a synergic effect s created due to this metal- ligand bonding. These synergic effects strengthen the bond between CO and the metal.
