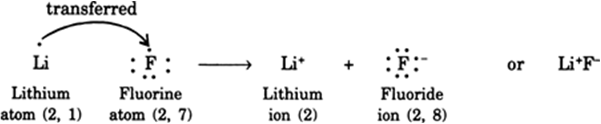
Give an account of the formation of lithium fluoride from its atoms.
A lithium atom can lose one electron easily to form positive lithium ion and a luoride atom can accept the electron released by lithium atom to form negative fluoride ion. Both the lithium ion and fluoride ion achieve noble gas configuration in this way and attract each other to form lithium fluoride. The total energy of the molecule is less than the sum of the energy of individual atoms involved.
93 Views

