 Multiple Choice Questions
Multiple Choice QuestionsCold ferrous sulphate solution on absorption of NO develops brown colour due to the formation of
paramagnetic [Fe(H2O)5(NO)] SO4
diamagnetic [Fe(H2O)5(N3)] SO4
paramagnetic [Fe(H2O)5(NO3)][SO4]2
diamagnetic [Fe(H2O)4 (SO4)]NO3
Which of the following option w.r.t. increasing bond order is correct ?
NO < C2 <O2- <He2+
C2 < NO < He2+ < O2-
He2+ < O2- < NO < C2
He2+ < O2- < C2 < NO.
The BCl3, is a planar molecule where as NCl3, is pyramidal. because
B-Cl bond is more polar than N-Cl bond
N-Cl bond is more covalent than B-Cl bond
nitrogen atom is smaller than boron atoms
BCl3 has no lone pair but NCl3 has a lone pair of electrons.
The electronic configuration is 1s2 2s2 2p5 3s1 shows
ground state of fluorine atom
excited state of fluorine atom
excited state of neon atom
excited state of ion O2
Assertion : B2H6, Si2H6, are said to have similar structure.
Reason : They have same number of and bonds.
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
If Assertion is true but Reason is false.
If Assertion is false but Reason is true.
D.
If Assertion is false but Reason is true.
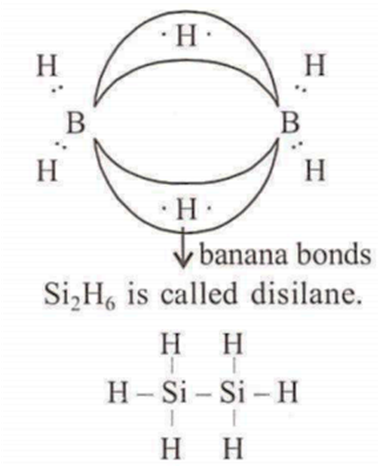
B2H6 is an electron deficient compound. B2H6 contain some unusual bonds which are called as 2-electron 3-centre bonds.
Assertion: Sigma () is a strong bond, while pi () is a weak bond.
Reason: Atoms rotate freely about the pi () bond.
If both assertion and reason are true and the reason is a correct explanation of the assertion.
If both the assertion and reason are true but the reason is not a correct explanation of the assertion.
If the assertion is true but the reason is false.
If both the assertion and reason are false.
The correct order of decreasing H-C-H angle in the following molecule is

I > II > III
II > I > III
III > II > I
I > III > II
The state of hybridization of the central atom and the number of lone pairs over the central atom in POCl3
are:
sp , 0
sp2 , 0
sp3 , 0
dsp2 , 1
The correct order of decreasing length of the bond as indicated by the arrow in the following structures is

I > II > III
II > I > III
III > II > I
I > III > II
