CBSE Multiple Choice Questions
Multiple Choice QuestionsElectrolysis of fused NaCl will give
Na
NaOH
NaClO
NaClO3
The effective neutron capture radius of a nucleus having a cross- section of 1.0 barr is [ Given, 1 barr = 1.0 x 10-24 cm2]
5.6 x 10-13cm
4.3 x 10-13cm
2.3 x 10-11 cm
5.6 x 10-24cm
When Br2 is treated with aqueous solutions of NaF, NaCl, NaI separately
F2, Cl2 and I2 are liberated
only F2 and Cl2 are liberated
only I2 is liberated
only Cl2 is liberated
If the for a given reaction has a negative value then which of the following gives the correct relationships for the values of G° and Keq?
< 0; Keq > 1
<0; Keq < 1
> 0; Keq < 1
> 0; Keq > 1
Which of the following statement is not correct about an inert electrode in a cell?
It does not participate in the cell reaction.
It provides surface either for oxidation or for reduction reaction.
It provides surface for conduction of electrons.
It provides surface for redox reaction
Choose the correct statement from the following.
The greater positive value of indicates greater reactivity of metal
F- is strong oxidant while Cu2+ is weak reductant
The metals placed above Mg in the electrochemical series do not decompose water at ordinary temperature
Oxides of Hg do not decompose on heating
Standard electrode potential of three metals X, Y and Z are -1.2 V, +0.5 V and -3.0 V respectively. The reducing power of these metals will be
Y > X > Z
Z > X > Y
X > Y > Z
Y > Z > X
Which of the following statement is correct?
Ecell and G of cell reaction both are extensive properties.
Ecell and of cell reaction both are intensive properties
Ecell in the intensive property while of cell reaction is an extensive property
Ecell is an extensive property while of cell reaction is an intensive property.
C6H5 - NHOH + C6H5 - NO (X), (X) formed in the above reaction is

(C6H5)2NOH
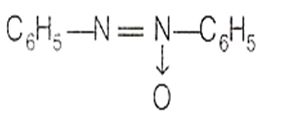
![]()
The conductivity of 0.001028 mol L-1 acetic acid is 4.95 x 10-5 S cm-1. Find out its dissociation constant if Λm for acetic acid is 390.5 S cm-1 mol-1.
2.18 x 10-5 mol-1L-1
1.78 x 10-5 mol L-1
3.72 x 10-4 mol L-1
2.37 x 10-4 mol L-1

