 Short Answer Type
Short Answer TypeThe elements Be, Mg and Ca each having two electrons in their outermost shells are in periods 2, 3, and 4 respectively of the modern periodic table. Answer the following questions, giving justification in each case:
(i) Write the group to which these elements belong.
(ii) Name the least reactive element.
(iii) Name the element having a largest atomic radius.
A carboxylic acid (molecular formula C2H402) reacts with an alcohol in the presence of an acid catalyst to form a compound 'X'. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid C2H402. Write the name and structure of
(i) carboxylic acid, (ii) alcohol and (iii) the compound 'X'.
Define the term ‘structural’ isomerism'. Explain why propane cannot exhibit this property. Draw the structures of possible isomers of butane, C4H10 .
A student wants to project the image of a candle flame on a screen 90 cm in front of a mirror by keeping the flame at a distance of 15 cm from its pole.
(a) Suggest the type of mirror he should use.
(b) Determine the linear magnification in this case.
(c) Find the distance between the object and its image.
(d) Draw ray diagram to show the image formation in this case.
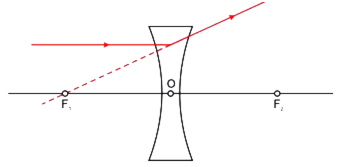
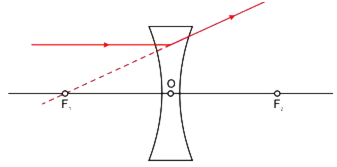
Draw a ray diagram to show the path of the refracted ray in each of the following cases:
A ray of light incident on a concave lens is:
(i) passing through its optical centre.
(ii) parallel to its principal axis.
(iii) directed towards its principal focus.
For a concave lens,
i) The ray diagram for a ray of light passing through the optical centre of the concave lens will emerge without any deviation.

A narrow beam PQ of white light is passing through a glass prism ABC as shown in the diagram.
Trace it on your answer sheet and show the path of the emergent beam as observed on the screen DE.
(i) Write the name and cause of the phenomenon observed.
(ii) Where else in nature is this phenomenon observed.
(iii) Based on this observation, state the conclusion which can be drawn about the constituents of white light.
