 Short Answer Type
Short Answer TypeWhat is the cause of a feeling of depression in human beings? Name a drug which can be useful in treating this depression.
Explain the following behaviours:
(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.
(ii) Ortho-nitro phenol is more acidic than ortho-methoxyphenol.
Explain the mechanism of acid catalysed hydration of an alkene to form corresponding alcohol.
Complete the following chemical reaction equations:
(i) C6H5N2Cl + H3PO2+ H2O --->
(ii) C6H5NH2+ Br2(aq.)--->
Describe the following giving the relevant chemical equation in each case:
(i) Carbylamines reaction
(ii) Hofmann’s bromamide reaction.
Answer the following question:
(i) What is meant by the chirality of a compound? Give an example.
(ii) Which one of the following compounds is more easily hydrolyzed by KOH and why?
CH3CHCICH2CH3 or CH3CH2CH2Cl
(iii) Which one undergoes S N 2 substitution reaction faster and why?![]()
Define the following as related to proteins:
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation
Differentiate between thermoplastic and thermosetting polymers. Give one example of each.
 Long Answer Type
Long Answer Type(a) Draw the molecular structure of the following compounds.
(i) N2O5
(ii) XeOF4
(b) Explain the following observation:
(i) Sulphur has a greater tendency for catenation than oxygen.
(ii) ICI is more reactive than I2.
(iii) Despite the lower value of its electron gain enthalpy with a negative sign, fluorine (F2) is a stronger oxidizing agent than Cl2.
i) 
ii)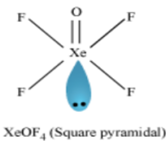
(b)
(i) Due to the small size of oxygen, it has less tendency for catenation and the high tendency of pp-pp multiple bonds, hence forms stable O2 molecules whereas sulphur because of its higher tendency for catenation and lesser tendency to form pp-pp multiple bonds forms S8 molecules having 8-membered puckered ring.
(ii)Inter -halogen bonds are weaker (it is between two different halogen like ICl) because of its partly ionic character due to the difference in electronegativity.
While when the same halogen forms X2 molecules like I2. They form covalent bonds which are stronger than interhalogen compound and weak bond obviously is more reactive than the stronger bond and that’s why ICl is more reactive than I2.
(iii) Fluorine is a much stronger oxidising agent than chlorine. The oxidising power depends on three factors.
1. Bond dissociation energy
2. Electron gains enthalpy
3. Hydration enthalpy
The electron gain enthalpy of chlorine is more negative than that of fluorine.
However, the bond dissociation energy of fluorine is much lesser than that of chlorine. Also, because of its small size, the hydration energy of fluorine is much higher than that of chlorine. Therefore, the latter two factors more than compensate for the less negative electron gain enthalpy of fluorine. Thus, fluorine is a much stronger oxidising agent than chlorine.
