 Short Answer Type
Short Answer TypeArrange the following in the decreasing order of their basic strength in aqueous solutions:
CH3NH2, (CH3)2NH, (CH3)3 N and NH3
A 1.00 molar aqueous solution of trichloroacetic acid (CCl3COOH) is heated to its boiling point. The solution has the boiling point of 100.180C. Determine the van’t Hoff factor for trichloroacetic acid. (Kb for water = 0.512 kg mol-1)
Define the following terms:
(i) Mole fraction
(ii) Isotonic solutions
(iii) Van’t Hoff factor
(iv) Ideal solution
(i) Mole fraction: The mole fraction of a component in a mixture is defined as the ratio of the number of moles of the component to the total number of moles of all the components in the mixture. Mathematically, it is represented as:
Mole fraction is denoted by ‘x’.
(ii) Isotonic solution: It is a type of solution that has the same salt concentration as its surrounding environment and thus the substances around it neither lose nor gain water by osmosis.
(iii) Van’t Hoff factor: It is defined as the ratio of the experimental value of colligative property to the calculated value of the colligative property and is used to find out the extent of dissociation or association.
Mathematically, it is represented as: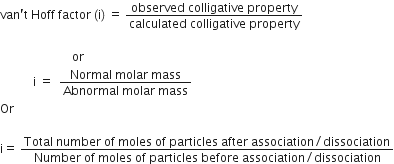
For association, i < 1
For dissociation, i > 1
No association or dissociation, i = 1
Examples: One formula unit of NaCl will create two particles in solution, a Na+ ion and a Cl- ion.
One formula unit of CaCl2 will create three particles in solution, a Ca+ ion and two Cl- ions.
(iv) Ideal Solutions: Ideal Solutions are those which obey Raoult's Law at all concentrations and Temperatures. Some examples of ideal solution liquid pairs are benzene and toluene, n-heptane and n-hexane, ethyl bromide and ethyl iodide, chlorobenzene and bromobenzene etc.
What do you understand by the ‘order of a reaction’? Identify the reaction order from each of the following units of reaction rate constant:
(i) L-1 mol-1
(ii) L mol-1 s-1
Name the two groups into which phenomenon of catalysis can be divided. Give an example of each group with the chemical equation involved.
What is meant by coagulation of a colloidal solution? Describe briefly any three methods by which coagulation of lyophobic sols can be carried out.
Describe the principle involved in each of the following processes.
(i) Mond process for refining of Nickel.
(ii) Column chromatography for purification of rare elements.
