 Short Answer Type
Short Answer Type(i) What is the role of t-butyl peroxide in the polymerization of ethene?
(ii) Identify the monomers in the following polymer:
OR
Write the mechanism of free radical polymerization of ethene.
Due to hectic and busy schedule, Mr. Angad made his life full of tensions and anxiety. He started taking sleeping pills to overcome the depression without consulting the doctor. Mr. Deepak, a close friend of Mr. Angad, advised him to stop taking sleeping pills and suggested to change his lifestyle by doing Yoga, mediation and some physical exercise. Mr. Angad followed his friend's advice and after few days he started feeling better.
After reading the above passage, Ans the following:
(i)What are the values (at least two) displayed by Mr. Deepak?
(ii)Why is it not advisable to take sleeping pills without consulting a doctor?
(iii)What are tranquillizers? Give two examples.
 Long Answer Type
Long Answer Typea) Calculate the freezing point of the solution when 1.9 g of MgCl2 (M = 95 g mol−1) was dissolved in 50 g of water, assuming MgCl2 undergoes complete ionization.
(Kf for water = 1.86 K kg mol−1)
(b)
(i) Out of 1 M glucose and 2 M glucose, which one has a higher boiling point and why?
(ii) What happens when the external pressure applied becomes more than the osmotic pressure of solution?
OR
(a)When 2.56 g of sulphur was dissolved in 100 g of CS2, the freezing point lowered by 0.383 K. Calculate the formula of sulphur (Sx).
(Kf for CS2 = 3.83 K kg mol−1, Atomic mass of sulphur = 32 g mol−1]
(b)Blood cells are isotonic with 0.9% sodium chloride solution. What happens if we place blood cells in a solution containing
(i)1.2% sodium chloride solution?
(ii)0.4% sodium chloride solution?
(a) Account for the following:
(i)Ozone is thermodynamically unstable.
(ii)Solid PCl5 is ionic in nature.
(iii)Fluorine forms only one oxoacid HOF.
(b) Draw the structure of
(i) BrF5
(ii) XeF4
OR
(i)Compare the oxidizing action of F2 and Cl2 by considering parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy.
(ii)Write the conditions to maximize the yield of H2SO4 by contact process.
(iii)Arrange the following in the increasing order of property mentioned:
(a)H3PO3, H3PO4, H3PO2 (Reducing character)
(b)NH3, PH3, AsH3, SbH3, BiH3 (Base strength)
a)
(i) Ozone decomposes into O2 with the evolution of heat, i.e. ΔH is negative (exothermic).
O3 --> O2 + O
ΔH = negative
Since the decomposition of O3 increases the number and freedom of particles, entropy also increases.
Therefore, DS = positive Now, ΔG = ΔH – TΔS
Both −∆H and
-T∆S(since ∆S is positive) result into large negative ∆G. Hence, O3 becomes thermodynamically unstable and decomposes into oxygen easily.
(ii) PCl5 is ionic in solid state because it exists as [PCl4]+ [PCl6]− in which the cation has tetrahedral geometry and the anion has octahedral geometry.
(b) (i) BrF5
(ii) XeF4
Or
(i) Although electron gain enthalpy of fluorine is less than that of chlorine because of the small size of fluorine, but the oxidising power depends on other factors like bond dissociation energy and hydration energy. The smaller the size of the atom, the greater the hydration enthalpy. Fluorine being small in size has higher hydration enthalpy as compared to chlorine.
Also, fluorine faces greater inter-electronic repulsion among its lone pairs of electrons because of its small size, while there is very less repulsion in chlorine. Hence, the bond dissociation enthalpy of fluorine is lower than that of chlorine.
Thus, the high hydration enthalpy and low bond dissociation enthalpy of fluorine result in its higher oxidising power as compared to that of chlorine.
(ii) Manufacturing of sulphuric acid via the contact process involves three steps:
(1) Burning of ores to form SO2
(2) Conversion of SO2 to SO3 using V2O5 as a catalyst
(3) Absorption of SO3 in H2SO4 to give oleum
The second step, i.e. conversion of SO2 to SO3 is the key step. Since this reaction is exothermic in nature and two moles of gaseous reactant give one mole of gaseous
Write the structures of A, B, C, D and E in the following reactions:
Or
(a)Write the chemical equation for the reaction involved in Cannizzaro reaction.
(b)Draw the structure of the semicarbazone of ethanal.
(c)Why pKa of F-CH2-COOH is lower than that of Cl−CH2−COOH?
(d)Write the product in the following reaction: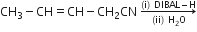
(e)How can you distinguish between propanal and propanone?
