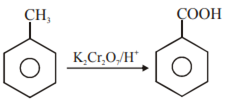Short Answer Type
Short Answer TypeWhen concentrated sulphuric acid was added to an unknown salt present in a test tube a brown gas (A) was involved. This gas intensified when copper turnings were added to this test tube. On cooling, the gas (A) changed into a colourless solid (B).
(i) Identify (A) and (B)
(ii) Write the structures of (A) and (B)
(iii) Why does gas (A) change to solid on cooling
Out of chlorobenzene and benzyl chloride, which one gets easily hydrolysed by aqueous NaOH and why?
How do you convert the following?
Toluene to Benzoic acid