 Short Answer Type
Short Answer TypeA first-order reaction is 50% completed in 40 minutes at 300 K and in 20 minutes at 320 K. Calculate the activation energy of the reaction.
(Given: log 2 = 0.3010, log 4 = 0.6021, R = 8.314 JK–1 mol–1)
What happens when
A freshly prepared precipitate of Fe(OH)3 is shaken with a small amount of FeCl3 solution?
Give reason:
When Cl2 reacts with an excess of F2. ClF3 is formed and not FCl3
Chlorine has empty d-orbital and it acquires excited state at the time of bonding when an electron from 3p-orbital are promoted to 3d- orbital.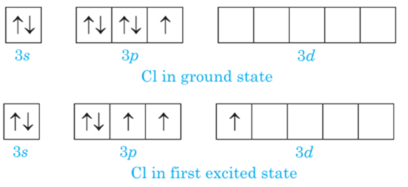
In first excited state chlorine atom can exhibit a covalency of three, hence cannot expand its octet due to the absence of empty d- orbitals in 2nd energy shell.
Hence, it cannot exhibit covalency more than 1therefore FCl3 is not possible.
