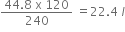Short Answer Type
Short Answer TypeWrite balanced chemical equation for the following reaction:
Zinc and dilute hydrochloric acid.
Mention the colour changes observed when the following indicators are added to acid:
Alkaline phenolphthalein solution.
Mention the colour changes observed when the following indicators are added to acid:
Methyl orange solution.
Mention the colour changes observed when the following indicators are added to acid:
Neutral litmus solution
Write balanced chemical equation for the following reaction:
Calcium bicarbonate and dilute hydrochloric acid.
Give one test each to distinguish between the following pairs of chemicals:
Zince nitrate solution and calcium nitrate solution.
Give one test each to distinguish between the following pairs of chemicals:
Sodium nitrate solution and sodium chloride solution
Give one test each to distinguish between the following pairs of chemicals:
Iron (III) chloride solution and copper chloride solution.
Dtermine the empirical formula of a compound containing 47.9 % potassium 5.5% beryllium and 46.6% fluorine by mass.
(Atomic weight of Be= 9;F=19;K-39) work to one decimal place.
Given that relative molecular mass of copper oxide is 80, what volume of ammonia (measured at stp) is required to competley reduce 120g of copper oxide?
The equation for the reaction is :
3CuO +2NH3 ---> 3Cu +3H2O +N2
(volume occupied by 1 mole of gas s.t.p is 22.4 litres)
3CuO + 2NH3 ---> 3Cu + 3H2O + N2
3mole 2mole
3x80 2 x 22.4
=240 =44.8l
120g ?