 Short Answer Type
Short Answer TypeWrite the equation for the following reaction:
Magnesium sulphate solution is mixed with barium chloride solution.
Write the equation for each of the following reaction;
zinc oxide is treated with sodium hydroxide solution.
LPG stands for liquefied petroleum gas. Varieties of LPG are marketed including a mixture of propane (60%) and butane (40%). If 10 litre of this mixture is burnt find the total volume of carbon dioxide gas is added to the atmosphere. Combustion
C3H8(g) +5O2(g)----> 3CO2(g) +4H2O(g)
2C2H10(g) +13O2(g) -------> 8CO2(g) +10H2O (g)
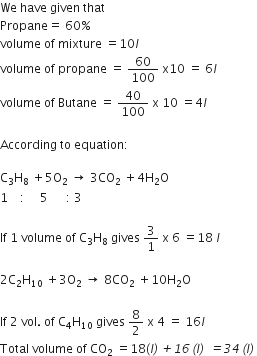
Calculate the percentage of nitrogen and oxygen in ammonium nitrate. (relative molecular mass of ammonium nitrate is 80, H=1, N=14, O=16.)
Three different electrolytic cells A, B, and C are connected in separate circuits. Electrolytic cell A contains sodium. When the circuit has completed a bulb in the circuit glows brightly. Electrolytic cell B contains an acetic acid solution and in this case, the bulb in the circuit glows dimly. The electrolytic cell C contains sugar solution and the bulb does not glow. Give a reason for each of this observation.
State your observation for the following cases:
Glass rod dipped in ammonium hydroxide is brought near the mouth of the concentrated hydrochloric acid bottle.
The diagram shows simple arrangement of the fountain experiment:

(i)Name the two gases you have studied which can be used in this experiment.
(ii) What is the common property demonstrated by this experiment?
