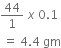Short Answer Type
Short Answer TypeWhat would you observe in the following case?
Sugar crystal is added to a hard glass test tube containing concentrated sulphuric acid.
What happens to the crystals of washing soda when exposed to air? Name the phenomenon exhibited.
What would you observe in the following case?
Ammonium hydroxide is first added in a small quantity and then in excess to a solution of copper sulphate.
Write the balanced chemical equation for the following reaction:
Zinc is heated with sodium hydroxide solution.
Write balanced chemical equation for the following:
Zinc oxide dissolves in sodium hydroxide
Calculate the volume of oxygen required for the complete combustion of 8.8g of propane (C3H8).
An organic compound with vapour density=94 contains C =12.67%, H=2.13% and Br=85.11%. Find the molecular formula,[Atomic mass: C=12, H=1, Br=80]
Calculate the mass of :
i) 1022atoms of sulphur
ii) 0.1 Mole of carbon dioxide
[Atomic mass : S =32.C=12 and O =16 and Avogadro's number=6x1023]
i) If 6 x 1023 atoms of sulphur =32
Then, 1022 atoms of sulphur =
ii) If 1 mole of CO2 =44 gm
Then, 0.1 mole of CO2 =