 Short Answer Type
Short Answer TypeWrite balanced equation for the following :
Preparation of ethane from sodium propionate.
Distinguish between the following pair of compound using the test given with bracket:
Ethane and ethane (using alkaline potassium permanganate solution).
state the conditions required for the following reactions to take place.
Catalytic hydrogenation of ethyne.
State the conditions required for the following reactions to take place.
Preparation of ethyne from ethylene dibromide.
Give balanced equation for the following:
Preparation of ethanol from monochloroethane and aqueous sodium hydroxide.
Q4. (a) give the structural formula of the following:
i) Ethanol
ii) 1-propananl
iii) Ethanoic acid
iv) 1,2 dichloroethane.
 Long Answer Type
Long Answer Typeb) M is a metal above hydrogen in the activity series and its oxide has the formula M2O, This oxide when dissolved in water forms the corresponding hydroxide which is a good conductor of electricity. In the above context answer the following:
i) what kind of combination exist between M and O?
ii) How many electrons are there in the outermost shell of M?
iii) Name the group of which M belongs.
iv) State the reaction taking place at the cathode.
v) Name the product at the anode.
A cylinder contains 68g of ammonia gas at s.t.p.
1) What is the volume occupied by this gas?
2) How many moles of ammonia are present in the cylinder?
3)How many molecules of ammonia are present in the cylinder?
[N-14,H-1]
Study the figure below and answer the question that follows: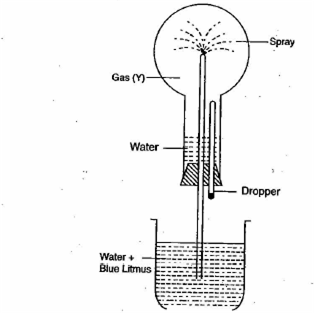
(i) Identify the gas Y
(ii) What property of gas Y does this experiment demonstrate?
(iii) Name another gas which has the same property and can be demonstrated through this experiment.
i) Hydrogen chloride gas (HCl).
ii) Y gas i.e, HCl gas is highly soluble and acidic in nature.
iii) Ammonia gas
