 Short Answer Type
Short Answer TypeIdentify the term/substance in each of the following:
The tendency of an atom to attract electrons to itself when combined with a compound.
Identify the term/substance in each of the following:
The method used to separate ore from gangue by preferential wetting.
Identify the term/substance in each of the following:
The catalyst used in the conversion of ethyne to ethane.
Identify the term/substance in each of the following:
The type of reactions alkenes undergo.
Identify the term/substance in each of the following:
The electrons present in the outermost shell of an atom.
i) A gas of mass 32 gram has a volume of 20 litres at S.T.P. Calculate the gram molecular weight of the gas.
ii) How much Calcium oxide is formed when 82g of calcium nitrate is heated? Also find the volume of nitrogen dioxide evolved:
2Ca(NO3)2 --> 2CaO +4NO2+ O2
(Ca =40, N=14, O=16)
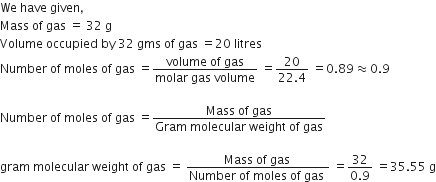
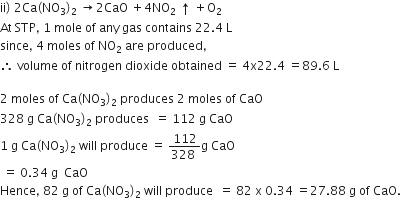
 Match The Following
Match The FollowingMatch the salts given in Column I with their method of preparation given in Column II:
| A. Pb(NO3)2 from PbO | (i) Simple displacement |
| B. MgCl2 from Mg | (ii) Titration |
| C. FeCl3 from Fe | (iii) Neutralization |
| D. FeCl3 from Fe | (iv) Precipitation |
| E. ZnCO3 from ZnSO4 | (v) Combination |
 Short Answer Type
Short Answer TypeRewrite the following sentences by using the correct symbol > (greater than) or < (less than) in the blanks given:
1. The ionization potential of Potassium is _______ that of Sodium.
2. The electronegativity of Iodine is _______ that of Chlorine.
Use the letter only written in the periodic Table given below to answer the questions that follow:
i) State the number of valence electrons in atom J.
ii) Which element shown forms ions with a single negative charge?
iii) Which metallic element is more reactive than R?
iv) Which element have its electrons arranged in four shells?
