 Fill In the Blanks
Fill In the BlanksFill in the blanks by selecting the correct word from the brackets:
i) If an element has a low ionisation energy then it is likely to be ______ (metallic/non-metallic).
ii) If an element has seven electrons in its outermost shell then it is likely to have the _______(largest/smallest) atomic size among all the elements in the same period.
 Short Answer Type
Short Answer TypeThe following table shows the electronic configuration of the elements W, X, Y, Z:
| Element | W | X | Y | Z |
| Electronic configuration | 2,8,1 | 2,8,7 | 2,5 | 1 |
Answer the following question based on the above:
(i) What type of Bond is formed between:
1. W and X
2. Y and Z
(ii) What is the formula of the compound formed between:
1. X and Z
2. W and X
(i)
(1) The Ionic bond is formed by transfer of one electron from element W to element X.
(2) A covalent bond is formed by sharing of electrons between elements Y and Z.
(ii)
(1) HCl (hydrochloric acid) is formed between X and Z.
(2) NaCl(sodium chloride) is formed between W and X.
Write a balanced chemical equation for each of the following:
(i) Burning of ethane in a plentiful supply of air.
(ii) The action of Water on calcium carbide.
(iii) Heating of Ethanol at 1700C in the presence of Conc. Sulphuric acid.
Give the structural formulae of each of the following:
(i) 2-methyl propane
(ii)Ethanoic acid
(iii) Butan-2-ol
The equation for the reaction when compound A is bubbled through bromine dissolved in carbon tetrachloride is as follows: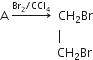
(i) Draw the structure of A.
(ii) State your observation during this reaction.
 Fill In the Blanks
Fill In the BlanksFill in the blanks using the appropriate words given below:
(sulphur dioxide, Nitrogen dioxide, Nitric oxide, Sulphuric acid).
i) Cold, dilute nitric acid reacts with copper to give__________.
ii) Hot, concentrated nitric acid reacts with sulphur to form________.
 Short Answer Type
Short Answer TypeIdentify the gas evolved and give the chemical test in each of the following cases:
i) Dilute hydrochloric acid reacts with sodium sulphite.
ii) The dilute hydrochloric acid reacts with iron (II) sulphide.
State your observations when ammonium hydroxide solution is added drop by drop and then in excess to each of the following solutions:
i) Copper sulphate solution
ii) Zinc sulphate solution.
Write equations for the reactions taking place at the two electrodes (mentioning clearly the name of the electrode) during the electrolysis of:
i) Acidified copper sulphate solution with copper electrodes.
ii) Molten lead bromide with inert electrodes.
i) Name the product formed at the anode during the electrolysis of acidified water using platinum electrodes.
ii) Name the metallic ions that should be present in the electrolyte when an article made of copper is to be electroplated with silver.
