 Short Answer Type
Short Answer TypeAn aqueous solution of sodium sulphate is acidic in nature.
The solubility product of silver chloride in water decreases on the addition of potassium chloride to the solution.
We have given,
Molecular weight of Y = 60
Osmotic pressure of solution of Y is 5 times the solution containing X.
Let molecular weight x = Mx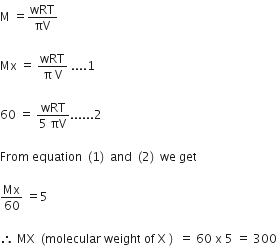
Which of the following solutions will have a lower vapour pressure and why ?
(1) A 5% solution of cane sugar (CI2H22O11)
(2) A 5% solution of urea (NH2CO NH2)
(Relative atomic masses: H = 1, C = 12, 0 = 16, N = 14).
(i) In a body centred and face centred arrangement of atoms of an element, what will be the number of atoms present in respective unit cells?
(ii) Explain why graphite is soft and can be used an a lubricant.
