 Short Answer Type
Short Answer Type(i) What is meant by promoter? Give an example.
(ii) The solubility product of BaS04 is 1.5 x 10-9. Find out its solubility in pure water. [1]
(iii) What is the discociation constant of 0.1M solution of a week add HA which is 4.5% ionized at 20°C
i) The promoter is a substance that increases the activity of the catalyst, for example, Mo act as a promoter in Haber’s process to increase the activity of Fe.
ii) 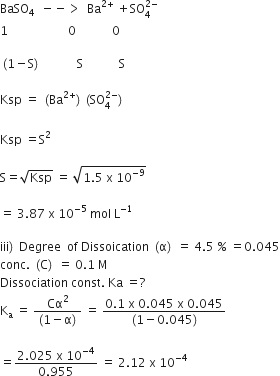
For the complex -ion of [Fe(CN)6]-3:
(i) Show the hybridization diagrammatically.
(ii) Is it an inner orbital complex or an outer orbital complex?
(iii) State its magnetic property.
Give balanced the chemcial equations for the following:
(i) Chlorine gas is passed through cold, dilute NaOH.
(ii) Sulphur dioxide gas is passed through NaOH solution.
(iii) Zinc is added to sodium agrentocyanide solution.
