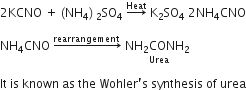Short Answer Type
Short Answer TypeGive one good chemical test to distinguish between the following pairs of compounds:
(i) Methanal and ethanal.
ii) Urea and benzoic acid
An aliphatic hydrocarbon Aon treatment with sulphuric acid in the presence of HgSO4 yields a liquid B with molecular formula C2H40. B on oxidation with acidified potassium dichromate yields C which gives effervescence with sodium bicarbonate. C when treated with SOCl2gives D. When D reacts with ethanol it gives a sweet smelling liquid E. E is also formed when C reacts with ethanol in the presence of conc. H2S04.
(i) Identify A, B, C, D and E.
(ii) Draw the structure of the isomer of compound B.
(iii) Write the balanced equation for the conversion of A to B.
(i)The compound C6Hl2 shows optical isomerism. Draw the structural formula of the compound and name it.
(ii) Name any three types of isomerisms that the compound with molecular formula C4H7Cl can give rise to. Also, represent the structures of the compounds relevant to these isomers.