 Short Answer Type
Short Answer Typei) Define molar conductance of a solution. State its unit. How is it related to the specific conductance of a solution ?
ii) Calculate the value of Ecell at 298K for the following cell:
Al|Al3+ (O.OIM)|| Sn2+(0.015M)|Sn
E° Al+3/Al = -1.66 volt and [E0 sn2+/Sn, = -0.14 volt]
(i) Calculate the degree of hydrolysis of 0.2(M) sodium acetate solution.
(Hydrolysis constant of sodium acetate = 5.6x 10 -10 and ionic product of H20 = 10 -14 at 25°C )
(ii) Explain why high pressure is used in the manufacture of ammonia by Haber’s process. State the law or principle used.
Give the IUPAC names of the following coordination compounds :
(i) K2[Zn(OH)4]
(ii) [Co(NH3)5(Co3)]Cl
For the complex ion [Fe(CN)6]3- state :
(i) The geometry of the ion.
(ii) The magnetic property of the ion.
For the molecule XeF2.
(i) Draw the structure of the molecule indicating the lone pairs.
(ii) State the hybridisation of the central atom.
(iii) State the geometry of the molecule.
i) 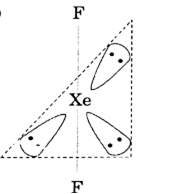
ii) Sp3d
(iii) Linear
Give balanced chemical equations for the following reactions:
(i) Fluorine treated with dilute sodium hydroxide solution.
(ii) Hydrogen sulphide treated with concentrated sulphuric acid.
(iii) Potassium iodide treated with acidified potassium permanganate solution.
In the extraction of zinc from zinc how oxide is converted to zinc.
(i) Give an equation to show how zinc oxide is converted to zinc.
(ii) How is impure zinc finally electro-refined.
Explain why:
(i) Transition elements form coloured compounds.
(ii) Interhalogen compounds are more reactive than their constituent elements.
(iii) Cu+ is diamagnetic but Cu2+ is paramagnetic. (Z = 29).
How can the following conversions be brought about:
(i) Nitro benzene to benzene diazonium chloride.
(ii) Propanoic acid to ethylamine.
(iii) Benzoic acid to benzaldehyde
