 Short Answer Type
Short Answer TypeHow can the following conversions be brought about:
(i) Acetic acid to methyl cyanide.
(ii) Acetaldehyde to formaldehyde.
(iii) Nitrobenzene to 2, 4, 6 tribromoaniline.
The deficiency of which vitamin will cause the following diseases:
(i) Scurvy
(ii) Haemorrhages
Give one chemical test to distinguish between the following pairs of compounds:
(i) Ethanol and 2 propanol.
(ii) Aniline and ethylamine
(i) Ethanol and propan-2-ol :
Lucas Test – With concentrated HCl + ZnCl2 ethanol no turbidity is formed.
Turbidity appears within five minutes in propan-2-ol.
(ii) Aniline and ethylamine:
Azo dye Test – Dissolve the given amine in dil. HCl and cool it in ice-cold water. Now add ice-cold solution of NaNO2 and HCl followed by an ice-cold β-naphthol solution. The appearance of brilliant orange-red indicates that the given amine is aniline.
Ethylamine does not form any dye and therefore does not show any colour when treated similarly.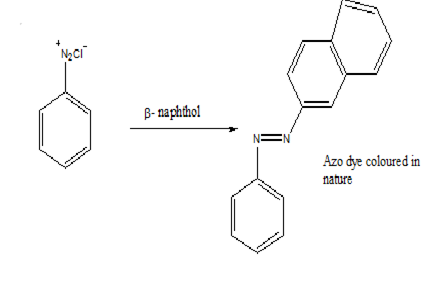
Give balanced equations for the following reactions:
(i) Acetaldehyde is heated with hydroiodic acid in the presence of red phosphorous.
(ii) Calcium acetate is subjected to dry distillation.
(iii) Sodium ethoxide is treated with ethyl bromide.
(iv) Benzaldehyde is treated with sodium bisulphite.
An organic compound A with molecular formula C7H8 on oxidation by chromylchloride in the presence of CCl4 gives a compound B which gives positive tollen’s test. The compound B on treatment with NaOH followed by acid hydrolysis gives two products C and D. C on oxidation gives B which on further oxidation gives D. The compound D on distillation with soda lime gives a hydrocarbon E. Below 60oC, concentrated nitric acid reacts with E in the presence of concentrated sulphuric acid forming a compound F. Identify the compounds A, B, C, D, E and F.
Give balanced equations for the following name reactions:
(i) Clemmensen’s reduction.
(ii) Kolbe’s electrolytic reaction.
(iii) Balz-Schiemann’s reaction.
(i) What do you observe when glucose is treated with bromine water?
(ii) What is isoelectric point?
Answer the following:
(i) What is biuret test?
(ii) Write balanced equation for the formation of biuret.
