 Short Answer Type
Short Answer TypeHow will you obtain pure potassium permanganate (KMnO4) crystals from its ore, pyrolusite? Give the step involved and the reactions.
Step I: Conversion of pyrolusite ore (MnO2) into potassium manganate.
The finely powdered pyrolusite mineral (MnO2) is fused with potassium hydroxide or potassium carbonate in the presence of air or oxidising agent such as potassium nitrate or potassium chlorate giving green coloured potassium manganate.
The reaction mixture containing K2MnO4 (potassium manganate) is treated with water and then converted
into KMnO4 (potassium permanganate) either by oxidation or by electrolysis.
Step II: Oxidation of potassium manganate (K2MnO4) to potassium permanganate (KMnO4).
![]()
For the reaction: 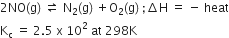
what will happen to concentration of N2 if:
i) Temperature is decreased to 273K
ii) Pressure is reduced.
What will be the value of van't Hoff factor (i) Of benzoic acid if it dimerised in aqueous solution? How will the experimental weight vary as compared to the normal molecular weight?
