 Short Answer Type
Short Answer TypeFor the complex ion [Fe(CN)6]3-, state:
(i) The type of hybridisation
(ii) The magnetic behaviour
(iii) The oxidation number of the central metal atom
The electronic configuration of Fe is Ar[18] 4s2 3d6
The electronic configuration of Fe3+ is Ar[18]3d5 4s0
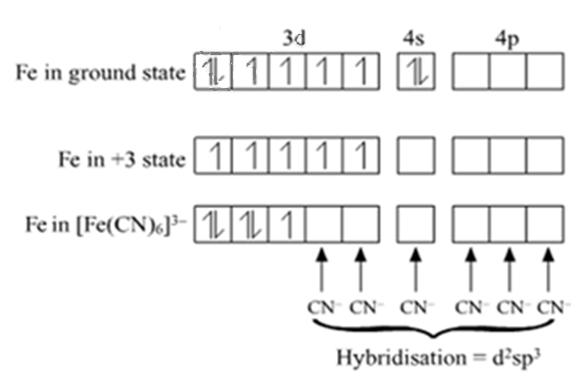
Hybridisation: d2sp3
Magnetic character: Paramagnetic
Spin nature of complex: Low-spin complex
The oxidation number of Fe is (+3) in this complex
Explain why :
Mn2+ is more stable than Fe2+ towards oxidation to +3 state. (At. no. of Mn = 25, Fe = 26)
Arrange the following in the increasing order of their basic strength:
C2H5NH2, C6H5NH2, (C2H5)2NH
What happens when benzene diazonium chloride reacts with phenol in weak alkaline medium? (Give balanced equation).
