 Multiple Choice Questions
Multiple Choice QuestionsThe electronic configuration of elements A, B and C are [He] 2s1, [Ne] 3s1 and [Ar] 4s1 respectively, which one of the following order is correct for the first ionization potentials (in kJ mol-1) of A , B and C?
A > B > C
C > B > A
B > C > A
C > A > B
The catenation tendency of C, Si and Ge is in the order Ge < Si < C. The bond energies (in kJ mol-1) of C-C, Si-Si and Ge-Ge bonds, respectively are
167, 180, 348
180, 167, 348
348, 167, 180
348, 180, 167
Ionic radius (in Å) of As3+, Sb3+ and Bi3+ follow the order
As3+ > Sb3+ > Bi3+
Sb3+ > Bi3+ > As3+
Bi3+ > As3+ > Sb3+
Bi3+ > Sb3+ > As3+
The number of lone pairs of electrons present on : Xe in XeF4 is
3
4
1
2
D.
2
Xe in ground state → 5s2, 5p6, 5d0
Xe in IInd excited state → 5s2, 5p4, 5d2
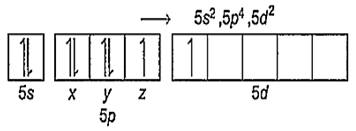
Number of lone pairs = 2
Number of bond pairs = 4
Therefore, the number of lone pairs of electrons present on Xe in XeF4 are 2.
The energy of an electron present in Bohr's second orbit of hydrogen atom is
-1312 J atom-1
-328 kJ mol-1
-328 J mol-1
-164 kJ mol-1
In the ground state, an element has 13 electrons in M shell. The element is
copper
chromium
nickel
iron
In a nuclide, one a.m.u. of mass is dissipated into energy to bind its nucleons. The energy equivalent of this mass is
931.5 eV
931.5 × 106 eV
931.5 × 106 MeV
931.5 MV
Let electronegativity, ionisation energy and electron affinity be represented as EN, IP and EA respectively. Which one of the following equation is correct according to Mulliken?
EN = IP × EA
EN =
EN = IP - EA
