Multiple Choice Questions
Multiple Choice QuestionsHow many litres of oxygen (at STP) are required for complete combustion of 39 g of liquid benzene? (Atomic weights: C = 12, O = 16, H = 1).
84
22.4
42
11.2
In which of the following reactions, H2O2 acts as a reducing reagent?
PbO2(s) + H2O(aq) → PbO(s) + H2O(l) + O2(g)
Na2SO3(aq) + H2O2(aq) → Na2SO4(aq) + H2O(l)
2KI(aq) + H2O2(aq) → 2KOH(aq) + I2(s)
KNO2(aq) + H2O2(aq) → KNO3(aq) + H2O(l)
In organic reactions, sodium in liquid ammonia is used as
reducing agent
hydrating agent
oxidising agent
precipitating agent
Which one of the following is mainly responsible for depletion of ozone layer?
Methane
Cabon dioxide
Water
Chloro-fluoro carbons
The structural formula of 2-methyl-2-butene is
CH3-CH(CH3)-CH=CH2
CH3-CH2-C(CH3)=CH2
CH3-CH=CH-CH3
CH3-CH=C(CH3)-CH3
Which one of the following pairs of compounds are functional isomers?
CH3CH2CH2OH, (CH3)2CHCH2OH
CH3CH2CH2CH2OH, (CH3)2CHCH2OH
CH3CH2CH2OH, CH3CH2CH2Cl
CH3CH2CH2OH, CH3-O-CH2CH3
What is Y in the following reaction?
C2H5I + NaOC2H5 → X + NaI
X + 2HHI 2Y + H2O
C2H6
C2H5I
C2H4
C2H5OC2H5
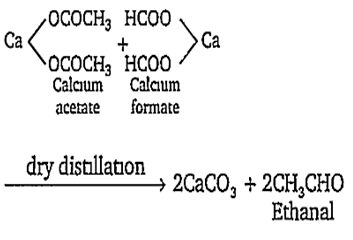
Dry distillation of calcium acetate and calcium formate forms:
methanol
ethanal
ethanol
acetone
B.
ethanal
Dry distillation of calcium acetate and calcium formate forms ethanal.