 Multiple Choice Questions
Multiple Choice QuestionsX litre of carbon monoxide is present at STP. It is completely oxidized to CO2. The volume of CO2 formed is 11.207 litres. What is the value of X in litres?
22.414
11.207
5.6035
44.828
The bond energies (in kJ mol-1) of P-H; As- H and N-H are respectively
247, 389 and 318
247, 389 and 318
318, 389 and 247
318, 247 and 389
Which one of the following represents the correct order of electronegativity ?
P > O > N
N > P > O
O > N > P
N > O > P
4 g of an ideal gas occupies 5.6035 L of volume at 546 K and 2 atmosphere pressure. What is its molecular weight?
4
16
32
64
Which one of the following statements is correct with respect to basic character ?
PH3 > P(CH3)3
PH3 = NH3
PH3 > NH3
P(CH3)3 > PH3
The calculated magnetic moment (in Bohr magnetons of Cu2+ ion is)
1.73
zero
2.6
3.4
A.
1.73
Electronic configuration of Cu2+ is-
1s2, 2s2, 2p6, 3s2, 3p6, 3d9 (because 2e- goes from 4s orbital)
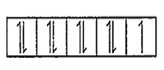
Number of unpaired electrons = 1
Magnetic moment =
=
=
= 1.732
Therefore, 1.732 is the magnetic moment of Cu2+.
75 mL of 0.2 M HCl is mixed with 25 mL of 1M HCl. To this solution, 300 mL of distilled water is added. What is the pH of the resultant solution?
1
2
4
0.2
The concentration of a 100 mL solution containing X g of Na2CO3 (molecular wt. = 106) is Y M. The values of X and Y are respectively.
2.12, 0.05
1.06, 0.2
1.06, 0.1
2.12, 0.1
One mole of fluorine is reacted with two moles of hot concentrated KOH. The products formed are KF, H2O and O2. The molar ratio of KF, H2O and O2 , respectively is
1 : 1 : 2
2 : 1 : 0.5
1 : 2 : 1
2 : 1 : 2
Which one of the following molecules contain both ionic and covalent bonds?
CH2Cl2
K2SO4
BeCl2
SO2
