 Multiple Choice Questions
Multiple Choice QuestionsIf an organic compound has C = 40%, H = 13.3% and N = 46.67%, then the empirical formula of this compound is
CH4N
C2H8N2
CH3N
None of these
The values for all the quantum numbers for 15th electron of chlorine are
n = 3; l = 1; m = 0; s = -
n = 4; l = 2; m = 0; s = +
n = 3; l = 1; m = +1; s = +
n = 2; l = 0; m = 0; s = +
C.
n = 3; l = 1; m = +1; s = +
Electronic configuration of chlorine is 17Cl - 1s2, 2s2 2p6, 3s2 3p5.
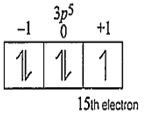
n = 3; l = 1; m = +1 and s = +
Which of the following is incorrect for radial distribution curve?
n = 2; l = 0; Node = 1
n = 3; l = 0; Node = 2
n = 2; l = 1; Node = 0
n = 3; l = 2; Node = 1
If H+ concentration of fruit juice is 3.3 × 10-2, then its pH will be
4.8 basic
4.8 neutral
4.8 acidic
1.6 acidic
If the value of bond order is zero, then
molecule will be stable
molecule will be unstable
molecule will be in ionic state
None of the above
Which is responsible for the diagonal relation of lithium with magnesium?
Less ionic radii
High polarising power
Approximately equal electronegativity and affinity
All of the above
Which of the following is a proper match?
| Shape | |
| A. 0.115 - 0.225 | i. Triangular |
| B. 0.225 - 0.414 | ii. Tetrahedral |
| C. 0.414 - 0.732 | iii. Cubic |
| D. 0.732 - 1 | iv. Octahedral |
A - i; B - ii; C - iv; D - iii
A - iii; B - ii; C - iv; D - i
A - i; B - iii; C - ii; D - iv
A - ii; B - iv; C - i; D - iii
