 Multiple Choice Questions
Multiple Choice QuestionsThe enthalpies of combustion of carbon and carbon monoxide are -393.5 and -283 kJ mol respectively. The enthalpy of formation of carbon monoxide per mole is
110.5 kJ
676.5 kJ
-676.5 kJ
-110.5 kJ
Which one of the following does not have sp2 hybridised carbon ?
Acetone
Acetic acid
Acetonitrile
Acetamide
The temperature dependence of rate constant (k) of a chemical reaction is written in terms of Arrhenius equation, k = Ae-E*/ RT. Activation energy (E*) of the reaction can be calculated by plating
log k vs
log k vs
k vs T
k vs
Formation of a solution from two components can be considered as
(1) pure solvent → separated solvent molecules, H1
(2) pure solute → separated solute molecules, H2
(3) separated solvent and solute molecules → solution, H3
Solution so formed will be ideal if
H3
Among the following, the pair in which the two species are not isostructural, is
SiF4 and SF4
IO and XeO3
BH
PF and SF6
A.
SiF4 and SF4
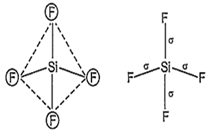
SiF4 and SF4 are not isostructural because SiF4 is tetrahedral due to sp3-hybridisation of Si.
14Si = 1s2, 2s2, 2p6, 3s2 3p2 (In ground state)
14Si = 1s2, 2s2, 2p6, 3s1 3p3 (In excited state)
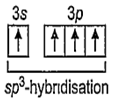
Hence, four equivalent sp3-hybrid orbitals are obtained and they are overlapped by four
p-orbitals of four fluorine atoms on their axes. Thus, it shows following structure:
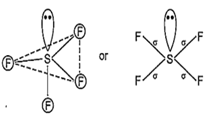
While SF4 is not tetrahedral but it is distorted tetrahedral because in it S is sp3d hybrid and has a lone pair of electron.
16S = 1s2, 2s2 2p6, 3s23p (In ground state)
= 1s2, 2s2 2p6, (In first excitation state)
Hence, sp3d hybrid orbitals are obtained. One orbital is already paired and rest four are overlapped with four p-orbitals of four fluorine atoms on their axes in trigonal bipyramidal form. This structure is distorted from trigonal bi-pyramidal to tetrahedral due to involvement of repulsion between lone pair and bond pair.
The maximum number of molecule is present in
15 L of H2 gas at STP
5 L of N2 gas at STP
0.5 g of H2 gas
10 g of O2 gas
Ionic radii are
inversely proportional to effective nuclear charge
inversely proportional to square of effective nuclear charge
directly proportional to effective nuclear charge
directly proportional to square of effective nuclear charge
The helical structure of protein is stabilized by
dipeptide bond
hydrogen bonds
ether bonds
peptide bonds
The radioactive isotope which is used in the treatment of cancer can be made by (n, p) reaction. For this reaction the target nucleus is
The work done during the expansion of a gas from a volume of 4 dm3 to 6 dm3 against a constant external pressure of 3 atm, is
-6 J
-608 J
+304 J
-304 J
