 Multiple Choice Questions
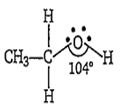
Multiple Choice QuestionsThe C-O-H bond angle in ethanol is nearly
90°
104°
120°
180°
A.
90°
In ethanol the oxygen of -OH group is bonded to the sp3 hybridised carbon by a sigma bond. The -C-O-H bond angle in ethanol is less than the tetrahedral angle (109° 28) due to larger repulsions between the lone pairs of repulsions between the lone pairs of oxygen. Hence it is 104° in ethanol.
A buffer solution is prepared by mixing 0.1 M ammonia and 1.0 M ammonium chloride. At 298 K, the pKb of NH4OH is 5.0. The pH of the buffer is
10.0
9.0
6.0
8.0
Among NH3, HNO3, NaN3 and Mg3N2 the number of molecules having nitrogen in negative oxidation state is
1
2
3
4
Born-Haber cycle may be used to calculate
electronegativity
mass number
oxidation number
electron affinity
The heat of formation for CO2 (g), H2O (l) and CH4 (g) are -400 kJ mol-1, -280 kJ mol-1 and -70 kJ mol-1 respectively. The heat of combustion of CH4 in kJ mol-1 is
890
-160
-890
-90
Oxygen and sulphur both are the member of same group in periodic table but H2O is liquid while H2S is gas because
molecular weight of water is more
electronegativity of sulphur is more
H2S is weak acid
water molecules are having weak hydrogen bonds between them
