Multiple Choice Questions
Multiple Choice QuestionsWhich of the following property does not correspond to the order HI < HBr < HCl < HF ?
Thermal stability
Reducing power
Ionic character
Dipole moment
The standard reduction potential for Fe2+/Fe and Sn2+/Sn electrodes are - 0.44 and- 0.14 V respectively. For the cell reaction, the standard emf is
+ 0.30 V
- 0.58 V
+ 0.58 V
- 0.30 V
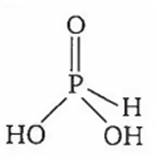
Which of the following phosphorus oxyacids can act as a reducing agent?
H3PO3
H3PO4
H2P2O6
H4P2O7
A.
H3PO3
Oxyacid of phosphorus which contains P-H bonds can act as a reducing agent. H3PO3 contains one P-H bond and hence acts as a reducing agent.