 Multiple Choice Questions
Multiple Choice QuestionsC6H6 + O3 → X Y; X and Y are respectively.
diozonide, glycol
triozonide, glyoxalic acid
triozonide, glyoxal
monoozonide, oxalic acid
If the equilibrium constant for the reaction,
2AB A2 + B2
is 49, what is the equilibrium constant for AB ?
7
24.5
49
What is the entropy change in JK-1 during the melting of 27.3 g of ice at 0°C? (Latent heat of fusion of ice = 330 Jg-1)
330
12.1
3.3
33
Identify the order in which the spin only magnetic moment (in BM) increases for the following four ions
(I) Fe2+
(II) Ti2+
(III) Cu2+
(IV) V2+
I, II, IV, III
IV, I, II, III
III, IV, I, II
III, II, IV, I
Which one of the following frequencies of radiation (in Hz) has a wavelength of 600 nm?
2.0 × 1013
5.0 × 1016
2.0 × 1014
5.0 × 1014
According to Bohr's theory which one of the following values of angular momentum of hydrogen atom is not permitted?
Which one of the following is correct order of second ionisation potential of Na, Ne, Mg and Al?
Al < Na < Mg < Ne
Ne < Al < Na < Mg
Mg < Al < Ne < Na
Na < Mg < Ne < Al
The formal charges of N(1), N(2) and O atoms in the following figure are respectively
![]()
+1, -1, 0
-1, +1, 0
+1, +1, 0
-1, -1, 0
In which of the following pairs, the central atoms have the same number of lone pairs of electrons?
PCl5, BrF5
XeF2, ICl
XeF4, ClO
SCl4, CH4
B.
XeF2, ICl
Both XeF2 and ICl molecule have three lone pairs of electron on central atom.

Hence, in XeF2 three lone pairs are present.
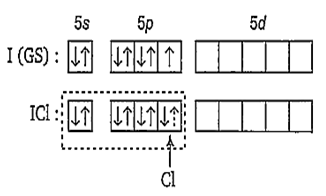
Hence, ICl three lone pairs are present.
