 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following oxides is reduced by water gas to obtain the metal during its extraction?
NiO
ZnO
WO3
Fe2O3
0.16 g of an organic compound containing sulphur produces 0.233 g of BaSO4. Percentage of sulphur in the compound is
20
80
50
10
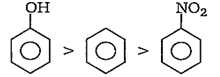
The order of reactivity of phenol (I), nitrobenzene (II) and benzene (III) towards nitration is
(III) > (I) > (II)
(II) > (III) > (I)
(I) > (III) > (II)
(I) > (II) > (III)
C.
(I) > (III) > (II)
Due to strong +R effect of -OH group, the electron density in phenol (I) is much more than in benzene (III) while due to the strong -R effect of the - NO2 group, the electron density in nitrobenzene (II) is much lower than in benzene. Hence, the order of reactivity towards nitration is as-
The pKa values of four carboxylic acids are 4.76, 4.19, 0.23 and 3.41 respectively. The pKa value ofstrongest carboxylic acid among them is
4.19
3.41
0.23
4.76
Which one of the following statements is not correct?
Except glycine all other naturally occurring α-amino acids are optically active.
α-amino acids have maximum solubility at their isoelectric point.
A tripeptide has two peptide bonds.
α-amino acids exist as Zwitter ions.
Which one of the following is true for an exothermic reaction A B, if Ef and Eb are the activation energies of forward and backward reactions respectively?
Ef > Eb
Ef = Eb
Ef = -Eb
Ef < Eb
Assertion (A) : Cyclohexane is the most stable cycloalkane.
Reason (R) : Cyclopropane and cyclobutane are less stable due to angle starin and torsional strain.
The correct answer is-
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true but (R) is not true.
(A) is not true but (R) is true.
Both (A) and (R) are true and (R) is the correct explanation of (A).
