 Multiple Choice Questions
Multiple Choice QuestionsThe solution of BiCl3 in dil. HCI when diluted with water, white precipitate is formed which is
Bismith oxychloride
Bismith oxide
Bismith hydroxide
None of the above
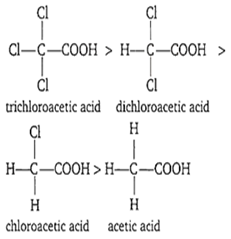
The strongest acid is
acetic acid
trichloroacetic acid
dichloroacetic acid
monochloroacetic acid
B.
trichloroacetic acid
Since, chloro group has -I effect. Due to which greater the number of electron withdrawing atoms (halogens), stronger will be the acid. Thus, order of acidity is
The degree of ionization of 0.4 M acetic acid will be (Ka = 1.8 × 10)
6.71 × 10-3
1.6 × 10-3
0.4 × 1.8 × 10-3
1.8 × 10-5
A solution contains Cl-, I- and SO ions in it. Which of the following ion is capable to precipitate all of the above when added in this solution?
Pb2+
Ba2+
Hg2+
Cu2+
