Multiple Choice Questions
Multiple Choice QuestionsA 100% pure sample of a divalent metal carbonate weighing 2 g on complete thermal decomposition releases 448 cc of carbon dioxide at STP. The equivalent mass of the metal is
40
20
28
12
The ratio of the frequency corresponding to the third line in Lyman series of hydrogen atomic spectrum to that of the first line in Balmer series of Li2+ spectrum is
The incorrectly matched pair, among the following is-
BrF5 - Trigonal bipyramidal
SF4 - See-saw
ClF3 - T-shape
NH3 - Trigonal pyramidal
Molecules/ ions and their magnetic properties are given below-
| Molecule/ Ion | Magnetic Property |
| i. C6H6 | 1. Antiferromagnetic |
| ii. CrO2 | 2. Ferrimagentic |
| iii. MnO | 3. Ferromagnetic |
| iv. Fe3O4 | 4. Paramagnetic |
| v. Fe3+ | 5. Diamagnetic |
The correctly matched pairs in the above is
i - 5; ii - 3; iii - 2; iv - 1; v - 4
i - 3; ii - 5; iii - 1; iv - 4; v - 2
i - 5; ii - 3; iii - 1; iv - 2; v - 4
i - 5; ii - 3; iii - 1; iv - 4; v - 2
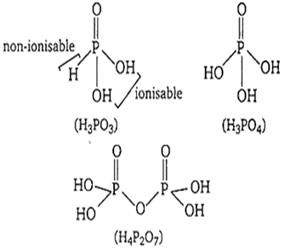
Choose the weak monobasic acid, among the following
H3BO3
H3PO3
H3PO4
HNO3
A.
H3BO3
H3BO3 is a weak Lewis monobasic acid, because it can only accept a pair of electron. None of its hydrogens are ionisable.