 Multiple Choice Questions
Multiple Choice QuestionsThe quantum number which explains the line spectra observed as doublets in case of hydrogen and alkali metals and doublets and triplets in case of alkaline earth metals is
spin
azimuthal
magnetic
principal
According to molecular orbital theory, the total number of bonding electron pairs in O2 is
2
3
5
4
Which one of the following equations represents the variation of viscosity coefficient (η) with temperature (T)?
η= Ae-E/RT
η= AeE/RT
η= Ae-E/kT
η= Ae-E/T
The number of moles of electrons required to deposit 36 g of Al from an aqueous solution of Al(NO3)3 is (At. wt. of Al = 27)
4
3
2
1
Which one of the following statements is not correct?
The pH of 1.0 ×10-8 M HCl is less than 7.
The ionic product of water at 25°C is 1.0 ×10-14 mol2L-2
Cl- is a Lewis acid
Bronsted Lowry theory cannot explain the acidic character of AlCl3.
Molar heat capacity (Cp) of water at constant pressure is 75 JK-1mol-1. The increase in temperature (in K) of 100 g of water when 1 kJ of heat is supplied to it is
2.4
0.24
1.3
0.13
With respect to chlorobenzene, which of the following statements is not correct ?
Cl is ortho/para directing
Cl exhibits + M effect
Cl is ring deactivating
Cl is meta directing
Match the following.
| Column I | Column II | ||
| A. | Acetaldehyde,vinyl alcohol | 1. | Enantiomers |
| B. | Eclipsed and staggered ethane | 2. | Tautomers |
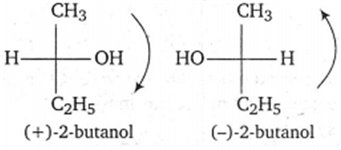
| C. | (+)2-butanol,(-)2-butanol | 3. | Chain isomers |
| D. | Methyl-n-propyl-amine and diethylamine | 4. | Conformational isomers |
| 5. | Metamers |
A B C D
1 4 3 5
A B C D
2 4 1 5
A B C D
5 1 4 2
A B C D
5 1 3 2
B.
A B C D
2 4 1 5
Thus, these two are tautomers.
Eclipsed and staggered ethane are two conformations of ethane.
(+) 2-butanol and (-)2-butanol are enantiomers as these are non-superimposable mirror images.
Due to difference in the nature of alkyl groups attached to the same functional group, these are called metamers.
