 Multiple Choice Questions
Multiple Choice QuestionsThe correct order of decreasing dipole moment of (I) toluene, (II) m-dichlorobenzene (III) o-dichlorobenzene and (IV) p-dichlorobenzene is
IV < II < I < III
IV < I < II < III
I < IV < II < III
IV < I < III < II
Which one of the following complexes is an outer orbital complex?
(Atomic number of Mn = 25; Fe = 26; Co = 27 and Ni = 28)
[Fe(CN)6]4-
[Mn(CN)6]4-
[Co(NH3)6]3+
[Ni(NH3)6]2+
For a gas in equilibrium with a liquid, the ratio of the concentration of the gas in the solution phase to that in the gaseous phase is constant at constant temperature, only if molecules undergo
ionisation
dissociation
isolation
reaction with solvent
In the following reaction, number of possible structure is
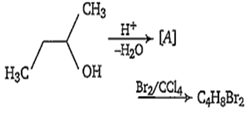
1
2
5
6
C.
5
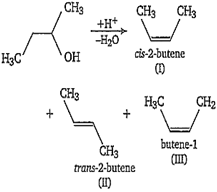
Therefore in X, order of stability of alkene is II > I > III.
All of these alkenes, with Br2/ CCl4, produce additive product having molecular formula C4H8Br2. The possible products are-
(i) CH3-CH(Br)-CH(Br)-CH3
(ii) CH3-CH(Br)-CH(Br)-CH3
(iii) BrH2C-CH(Br)-CH2-CH3
(iv) BrH2C-CH2-CHBr-CH3
(v) CH2Br-CH2-CH2-CH2Br
![]()
IUPAC name of the compound is
2-methyl-6-ethyloct-1, 5-dienal
3-ethyl-7-methyloct-2, 6-dienal
7-methyl-3-ethyloct-2, 6-dienal
None of the above
Iron pipes, lying in acidic soil, are often attached to the blocks of magnesium for their protection from rusting, because magnesium
is lighter than iron
is readily converted into positive ion
forms a corrosion-resistant alloy with iron
prevents air from reaching the surface of iron
The number of sodium atoms in 2 moles of sodium ferrocyanide is
12 × 1023
26 × 1023
34 × 1023
48 × 1023
Match the Column I and Column II and choose the correct code given below.
| Column I | Column II |
| A. Peroxyacetyl nitrile | i. Waste incineration |
| B. Indigo | ii. Vat dye |
| C. IR active molecules | iii. Global warming |
| D. Dioxins | iv. Photchemical smog |
A - iv; B - ii; C - iii; D - i
A - i; B - ii; C - iv; D - iii
A - ii; B - i; C - iv; D - iii
A - iv; B - i; C - iii; D - ii
The densities of graphite and diamond at 298 K are 2.25 and 3.31 g cm-3 respectively. If the standard free energy difference is 1895 J mol-1, the pressure at which graphite will be transformed into diamond is
9.92 × 108 Pa
9.92 × 107 Pa
9.92 × 106 Pa
None of these
