 Multiple Choice Questions
Multiple Choice QuestionsWhen 1 M H2SO4 is completely neutralized by NaOH, the heat liberated is 114.64 kJ. What is the enthalpy of neutralization?
+ 114.64 kJ
-114.64 kJ
-57.32 kJ
+ 57.32 kJ
A particle 'A' moving with a certain velocity has the de-Broglie wavelength 1 Å. For a particle 'B' with mass 25% of 'A' and velocity 75% of 'A'. The de-Broglie wave length of 'B' will be
3 Å
5.33 Å
6.88 Å
0.68 Å
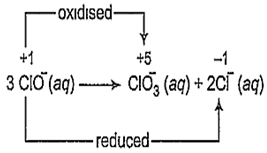
The reaction,
3ClO- (aq) → ClO (aq) + 2Cl- (aq) is an example of
oxidation reaction
reduction reaction
disproportionation reaction
decomposition reaction
C.
disproportionation reaction
A reaction in which the same species is simultaneously oxidised as well as reduced is called a disproportionation reaction. For such redox reactions to occur, the reacting species must contain an element which has atleast three oxidation states. The element in the reacting species is present in the intermediate oxidation state while the higher and lower oxidation states are available for reduction and oxidation to occur.
The geometrical and optical isomers of complex [Pt(NH3)(Br)(Cl)(Py)] respectively
2, 2
0, 3
2, 1
3, 0
One of the isomer of the 5th member of alkyne series is optically active. It is
4-methyl pent-2-yne
3-methyl pent-1-yne
4-methyl pent-1-yne
3, 3-dimethyl but-1-yne
Schiff's nitrometre is filled with
mercury
water over mercury seal
KOH solution over mercury seal
toulene over mercury seal
