 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following will have the shape of a trigonal bipyramid?
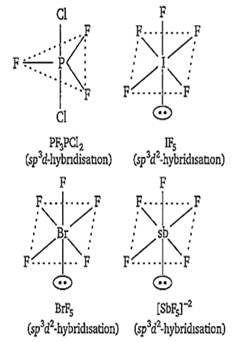
PF3Cl2
IF5
BrF5
SbF
A.
PF3Cl2
PF3Cl2 has trigonal bipyramidal structure, IF5 BrF5 and SbF have octahedral geometries with one position occupied by a lone pair of electrons (square pyramidal geometry).
5 millimoles of caustic potash and 5 millimoles of oxalic acid are mixed and dissolved in 100 mL water. The solution will be
basic
acidic
neutral
cannot say
How many moles of O2 can be obtained by electrolysis of 90 g H2O?
5.0 mol
0.5 mol
2.5 mol
0.25 mol
Four different sets of quantum numbers for 4 electrons are given below:
e1 = 4, 0, 0, -
e2 = 3, 1, 1, -
e3 = 3, 2, 2, +
e4 = 3, 0, 0, +
The order of energy of e1, e2, e3, e4 is
e1 > e2 > e3 > e4
e4 > e3 > e2 > e1
e3 > e1 > e2 > e4
e2 > e3 > e4 > e1
Electron affinity is positive when
O- is formed from O
O2- is formed from O-
O+ is formed from O
electron affinite is always a negative value
When solid melts, there will be
a decrease in enthalphy
a decrease in free energy
a decrease in entropy
all the above factors remain constant
Which solution is a buffer?
Acetic acid + NaOH (equimolar ratio)
Acetic acid + NaOH (1 : 2 molar ratio)
Acetic acid + NaOH (2 : 1 molar ratio)
HCl + NaOH (equimolar ratio)
