 Multiple Choice Questions
Multiple Choice Questionsd2sp3 hybridisation of the atomic orbitals gives
square planar structure
triangular structure
tetrahedral structure
octahedral structure
D.
octahedral structure
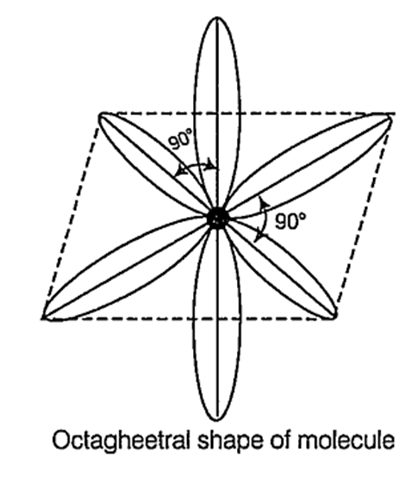
When are 5, three p and two d-orbItals belonging to the same shell of an atom mix together to form six new equivalent orbitals, the type of hybridisation is called d2sp3 or octahedral orbitals. The new orbitals are called d2sp3 or octahedral orbitals. Out of which four coplanar and two perpendicular to this plane.
A group of atoms can function as a ligand only when
It is a small molecule
It has an unshared electron pair
Its a negatively charged ion
It is a positively charge ion
The process is spontaneous at the given temperature, if
ΔH is +ve and ΔS is -ve
ΔH is -ve and ΔS is +ve
ΔH is +ve and ΔS is +ve
ΔH is +ve and ΔS is equal to zero.
Mesomeric effect involves
delocalisation of π- electron
delocalisation of σ- electron
partial displacement of electrons
delocalisation of π and σ- electron
Alkali metals have negative reduction potential and hence they behave as
oxidising agents
Lewis bases
reducing agents
electrolytes
Cycloalkane formed when 1,4-dibromopentaneis heated with sodium is
methyl cyclobutane
cyclopentane
cyclobutane
methyl cyclopentane
