 Multiple Choice Questions
Multiple Choice QuestionsIf a LPG cylinder contains mixture of butane and isobutane, then the amount of oxygen that would be required for combustion of 1 kg of it will be
2.50 × 103 gm
4.50 × 103 gm
180 × 103 gm
3.58 × 103 gm
The correct order of basic strength of the following are

1 > 2 > 3 > 4
4 > 2 > 3 > 1
3 > 4 > 2 > 1
3 > 2 > 4 > 1
An organic compound C3H5Cl (A) when treated with magnesium in dry ether gives (B) which on treating with CO2 followed by acid hydrolysis gives C4H6O2 (C). (C) is also obtained on oxidation of a hydrocarbon (D) C8H12. Structure of A is
CH2=CH-CH2-Cl
CH2=C(Cl)-CH3
CH(Cl)=CH-CH3
![]()
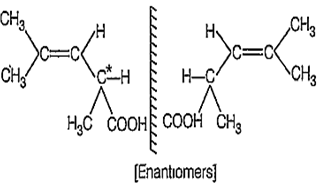
The structure shows

geometrical isomerism
optical isomerism
geometrical and optical isomerism
tautomerism
B.
optical isomerism
The compound does not show geometrical isomerism because one carbon atoms attached to double bond has same substituent group (-CH3), however, it can show optical isomerism, because it has one optically active chiral carbon atom.
What is the correct IUPAC name of

4-methoxy-2-nitrobenzaldehyde
4-formyl-3-nitroanisole
4-methoxy-6-nitrobenzaldehyde
2-formyl-5-methoxynitrobenzene
In Kjeldahl's method of estimation of nitrogen, CuSO4 act as
oxidising agent
reducing agent
catalytic agent
hydrolysis agent
The pairs of compounds which cannot exists together in a solution is
NaHCO3 and NaOH
Na2CO3 and NaOH
Na2CO3 and NaHCO3
NaHCO3 and NaCl
