 Multiple Choice Questions
Multiple Choice QuestionsFor which among the following equimolar aqueous solutions Van't Hoff factor has the lowest value?
Aluminium chloride
Potassium sulphate
Ammonium chloride
Urea
Which element among the following does not form diatomic molecules?
Argon
Oxygen
Nitrogen
Bromine
What is the quantity of hydrogen gas liberated when 46 g sodium reacts with excess ethanol? (Given atomic mass of Na = 23)
2.4 × 10-3 kg
2.0 × 10-3 kg
4.0 × 10-3 kg
2.4 × 10-2 kg
Tert-butyl methyl ether on treatment with hydrogen iodide in cold gives
tert-butyl iodide and methyl iodide
tert-butyl alcohol and methyl alcohol
tert-butyl alcohol and methyl iodide
tert-butyl iodide and methyl alcohol
D.
tert-butyl iodide and methyl alcohol
The reaction between tert-butyl methyl ether with hydrogen iodide is as follows-
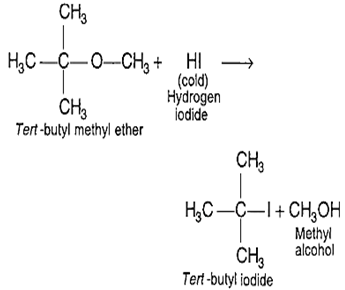
SN1 mechanism is followed as (CH3)3C+ is most stable carbocation and I- is attached to it.
In assigning R-S configuration, which among the following groups has highest priority?
-SO3H
-COOH
-CHO
-C6H5
Which of the following compounds has lowest boiling point?
n-butyl alcohol
lso-butyl alcohol
Tert-butyl alcohol
Sec-butyl alcohol
