Multiple Choice Questions
Multiple Choice QuestionsThe electronic configuration of 59Pr ( praseodimium) is
[54Xe] 4f25d16s2
[54Xe] 4f15d16s2
[54Xe] 4f36s2
[54Xe] 4f35d2
The bond lenght (pm) of F2, H2, Cl2 and I2, respectively is:
144, 74, 199, 267
74, 144, 199, 267
74, 267, 199, 144
144, 74, 267, 199
Which of the following corresponds to the energy of the possible excited state of hydrogen?
-13.6 eV
13.6 eV
-3.4 eV
3.4 eV
Which one of the following statement is correct for d4 ions [ P = pairing energy]
When Δ0 > P, low-spin complex form
When Δ0 < P, low-spin complex form
When Δ0 > P, high-spin complex form
When Δ0 > P, both high and low-spin complex form
Commercially available H2SO4 is 98 g by H2SO4 and 2g by weight of water. It's density is 1.38 g cm-3. Calculate the molality (m) of H2SO4 (molar mass of H2SO4 is 98 g mol-1).
500 m
20 molal
50 m
200 m
Standard enthalpy (heat) of formation of liquid water at 25°C is around
H2(g) + O2(g) → H2O (l)
-237 kJ/mol
237 kJ/mol
- 286 kJ/mol
286 kJ/mol
Given ΔHf° for CO2(g) , CO(g) and H2O(g) are -393.5, -110.5 and -241.8 kJ mol-1, respectively. The ΔHf° [in kJ mol-1] for the reaction
CO2(g) + H2(g) → CO(g) + H2O (g) is
524.1
-262.5
-417
412
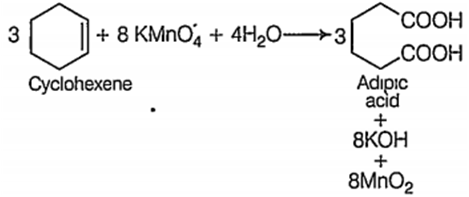
Oxidation of cyclohexene in presence of acidic potassium permaganate leads to
glutaric acid
adipic acid
pimelic acid
succinic acid
B.
adipic acid
Oxidation of cyclohexene in presence of acidic potassium permaganate leads to formation of adipic acid.