 Multiple Choice Questions
Multiple Choice QuestionsWhen metal ‘M’ is treated with NaOH, a white gelatinous precipitate ‘X’ is obtained, which is soluble in excess of NaOH. Compound ‘X’ when heated strongly gives an oxide which is used in chromatography as an adsorbent. The metal ‘M’ is
Fe
Zn
Ca
Al
The recommended concentration of fluoride ion in drinking water is up to 1 ppm as fluoride ion is required to make teeth enamel harder by converting [3Ca3 (PO4)2.Ca(OH)2] to
[3{Ca(OH)2}. CaF2]
[CaF2]
[3(CaF2). Ca(OH)2]
[3Ca3(PO4)2. CaF2]
For 1 molal aqueous solution of the following compounds, which one will show the highest freezing point?
[Co(H2O)3 Cl3].3H2O
[Co(H2O)6]Cl3
[Co(H2O)5Cl] Cl2.H2O
[Co(H2O)4Cl2]Cl.2H2O
How long (approximate) should water be electrolysed by passing through 100 amperes current so that the oxygen released can completely burn 27.66 g of diborane? (Atomic weight of B = 10.8 u)
1.6 hours
6.4 hours
0.8 hours
3.2 hours
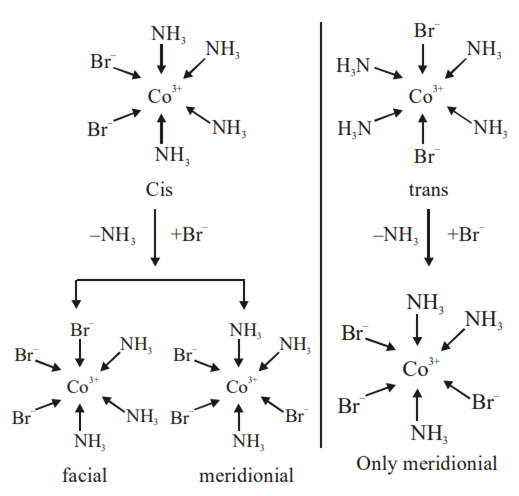
Consider the following reaction and statements:
(I) Two isomers are produced if the reactant complex ion is a cis-isomer
(II) Two isomers are produced if the reactant complex ion is a trans-isomer
(III) Only one isomer is produced if the reactant complex ion is a trans-isomer
(IV) Only one isomer is produced if the reactant complex ion is a cis – isomer
The correct statements are
(II) and (IV)
(I) and (II)
(I) and (III)
(III) and (IV)
C.
(I) and (III)
An aqueous solution contains an unknown concentration of Ba2+. When 50 mL of a 1 M solution of Na2SO4 is added, BaSO4 just begins to precipitate. The final volume is 500 mL. The solubility product of BaSO4 is 1 x 10–10. What is the original concentration of Ba2+?
1.0 x 10–10 M
5 x 10–9 M
2x10–9 M
1.1 x 10–9M
At 5180C the rate of decomposition of a sample of gaseous acetaldehyde initially at a pressure of 363 Torr, was 1.00 Torr s–1 when 5% had reacted and 0.5 Torr s–1 when 33% had reacted. The order of the reaction is
0
2
3
1
The trans-alkenes are formed by the reduction of alkynes with
Sn - HCl
H2 – Pd/C, BaSO4
NaBH4
Na/liq. NH3
Which type of ‘defect’ has the presence of cations in the interstitial sites?
Metal deficiency defect
Schottky defect
Vacancy defect
Frenkel defect
An aqueous solution contains 0.10 M H2S and 0.20 M HCl. If the equilibrium constants for the formation of HS– from H2S is 1.0 x10–7 and that of S2- from HS– ions is 1.2 x 10–13 then the concentration of S2- ions in aqueous solution is
5 x 10-19
5 x 10-8
3 x 10–20
6 x 10-21
