 Multiple Choice Questions
Multiple Choice QuestionsThe dimensions of in the equation where, p is pressure, x is distance and and t is time, are
[M2LT-3]
[MT-2]
[LT-3]
[ML3T-1]
Three vectors satisfy the relation A . B = 0 and A . C = 0, then A is parallel to
C
B
B × C
B . C
A student is standing at a distance of 50 metre from the bus. As soon as the bus begins its motion with an acceleration of 1 ms-2, the student starts running towards the bus with a uniform velocity u. Assuming the motion to be along a straight road, the minimum value of u, so that the student is able to catch the bus is
8 ms-1
5 ms-1
12 ms-1
10 ms-1
For a given velocity, a projectile has the same range R for two angles of projection if t1 and t2 are the time of flight in the two cases, then
t1t2 ∝ R
t1t2 ∝ R2
Weight of a body ofmass m decreases by 1 % when it is raised to height h above the earth's surface. If the body is taken to a depth h in a mine, change in its weight is
0.5 % decrease
2 % decrease
0.5 % decrease
1 % increase
Which of the following sets of concurrent forces may be in equilibrium ?
F1 = 3 N, F2 = 5 N, F3 = 1 N
F1 = 3 N, F2 = 5 N, F3 = 9 N
F1 = 3 N, F2 = 5 N, F3 = 6 N
F1 = 3 N, F2 = 2 N, F3 = 15 N
The displacement time graph of a particle executing SHM is as shown in the figure.

The corresponding force time graph of the particle is
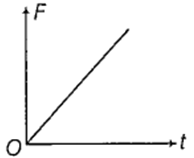
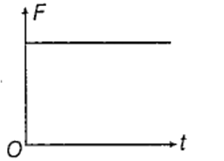


An ideal monoatomic gas at 27°C is compressed adiabatically to 8/27 times of its present volume. The increase in temperature of the gas is
375°C
402°C
175°C
475°C
A.
375°C
Given : T1 = 27 + 273 = 300 K
V1 = V (let)
Then for adiabatic process
For monoatomic gas,
Hence, increase in temperature
= 402°C − 27°C
= 375°C
A sample of ideal monoatomic gas is taken round the cycle ABCA as shown in the figure. The work done during the cycle is

3 pV
zero
9 pV
6 pV
