Multiple Choice Questions
Multiple Choice QuestionsAnhydrous ferric chloride is prepared by:
dissoving Fe(OH)3 in concentrated HCl
dissolving Fe(OH)3 in dilute HCl
passing dry HCl overheated iron scrap
passing dry Cl2 gas overheated iron scrap
Two aromatic compounds having formula C7H8O which are easily identifiable by FeCl3 solution test (violet colouration) are:
o-cresol and benzyl alcohol
m-cresol and p-cresol
o-cresol and p-cresol
methyl phenyl ether and benzyl alcohol
The ease of dehydrohalogenation of alkyl halide with alcoholic KOH is:
3° < 2° < 1°
3° > 2° > 1 °
3° < 2° > 1°
3° > 2° < 1 °
The correct order of decreasing acidity of nitrophenols will be:
m-nitrophenol > p-nitrophenol > o-nitrophenol
o-nitrophenol > m-nitrophenol > p-nitrophenol
p-nitrophenol > m-nitrophenol > o-nitrophenol
p-nitrophenol > o-nitrophenol > m-nitrophenol
In aqueous solution glucose remains as:
only in the open-chain form
only in the pyranose form
only in furanose form
in all three forms in equilibrium
The reaction of formaldehyde and ammonia gives:
hexamethylene tetramine
bakelite
urea
triethylene tetramine
Paracetamol is:
methyl salicylate
phenyl salicylate
N-acetyl p-amino phenol
acetyl salicylic acid
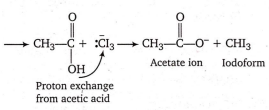
Which of the following compounds is not formed in iodoform reaction of acetone?
CH3COCH2I
ICH2COCH2I
CH3COCHI2
CH3COCl3
B.
ICH2COCH2I
Mechanism of iodoform reaction takes place by two steps:
(I) Iodination of -CO-CH3 group to form -CO-Cl3
CH3-CO-CH3 CH3-CO-CH2I + OH-
CH3-CO-CH2I CH3-CO-CHI2 + OH-
CH3-CO-CHI2 CH3-CO-CI3 + OH-
(II) Elimination of Cl3 as : CI3- anion