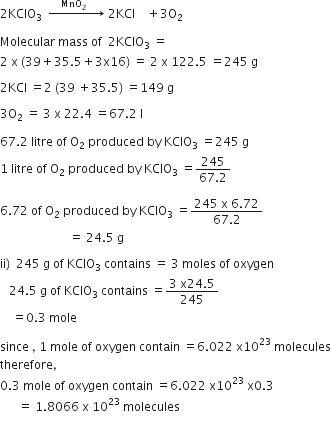
O2 is evolved by heating KClO3 using MnO2 as a catalyst

i) Calculate the mass of KClO3 required to produce 6.72 litres of O2 at STP.
[atomic masses of K =39, Cl=35.5, O=16]
ii) Calculate the number of moles of oxygen present in the above volume and also the number of molecules.
iii) Calculate the volume occupied by 0.01 mole of CO2 at STP