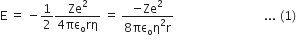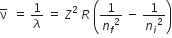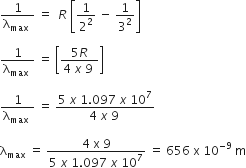From Bohr's energy quantization formula, determine the largest wavelength in the Balmer series of hydrogen atom spectrum.
From the Bohr’s energy equation,
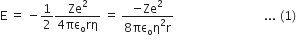
The wave number of radiation emitted by such as ion transition from ni to nf is given by,
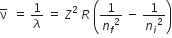
For Balmer Series :
ni = 3
nf = 2
219 Views